Marketing & Advertising
National Advertising Division found multiple faults with Oral Essentials’ evidence for claim, including that an in vitro study the firm conducted on “mouse cells is not dispositive of whether the same product would have any adverse effects on humans under real world conditions.”
“From heavy periods to painful cramps, the experiences of menstruation vary widely, but the need for understanding is universal," Maxwellia insists, as it launches a creative campaign addressing the confusion surrounding menstrual health in the UK. “With Evana driving momentum, menstrual health is finally getting the attention it deserves – one lipstick at a time.”
GuruNanda appealing a National Advertising Division decision, following a review of a Procter & Gamble challenge, that it cease using a “natural teeth whitening” claim for its namesake brand pulling oil, mouthwash and gel pen.
The trophies have been presented at the OTC Marketing Awards 2024 - but what did the judging panel have to say about the winning and highly-commended entries?
Winners across 12 categories were announced at the OTC Marketing Awards 2024 on 21 November, with Opella taking the top prize as OTC Company of the Year.
Opill marketer Perrigo builds on marketing collaboration with WNA in partnership with Napheesa Collier; Bucked Up partners with MMA champion and entrepreneur Conor McGregor; and Recover 180 collaborates with NBA players.
The International Council for Advertising Self-Regulation has launched a "Global Think Tank" to further effective industry self-regulation in the area of advertising, backed by national authorities such as the UK's ASA.
Reckitt backs up prediction for strong sales during current quarter with advertising featuring a beauty queen and a line extension for Mucinex, one its strongest US consumer health product lines.
Qnovia notes NRT inhalation product recently received investigational new drug clearance from FDA as agency and NIH say innovation needed smoking cessation to help improve rate of success for quitting the habit that kills around 500,000 US consumers annually.
Bayer adds dry eye treatment to its Bepanthen skin care range; Stada launches special edition Elotrans supplement for Eintracht Frankfurt fans; Hermes offers chewable magnesium supplement; and Klosterfrau introduces Oyono Night extension to help troubled sleepers maintain their immunity.
After launching Retaine MGD Advanced, OcuSoft says a release by Bruder Healthcare referenced Retaine MGD trademark and statements from a previous OcuSoft announcement about the original product attributed to an optometrist. B+L, Rohto brand and homeopathic firm Relief Products also make US OTC eye care space moves.
To coincide with Stoptober – an annual campaign that encourages smokers in the UK to quit smoking for the month of October – Kenvue has launched a digital “branded entertainment” series for smoking cessation brand Nicorette that follows smokers on their quitting journeys.
Maxwellia is inviting 100 investors to contribute to the “development of new healthcare solutions that will benefit millions, while also playing a role in shaping the future of self-care.”
Perelel acquiring Loom online platform providing reproductive health, sexual wellness and parenting education. First direct-to-consumer product for Curive Healthcare’s Plum brand formulated for vaginal area dryness.
Jessica Spence joins as North American president while CFO Dan Sullivan becomes COO and Francesca Weissman, finance and business strategy SVP, adds CFO to her title in latest executive office changes by the marketer of brands including Schick men's and women’s shaving, Playtex, Stayfree, Carefree and o.b. feminine care and Banana Boat and Hawaiian Tropic sun care.
Nonbinding forecast, fourth for the program, includes risks associated with codeine-containing cough medicine as a topic FDA will include in ongoing evaluation of GRASE for pediatric cough cold drug products marketed under monograph for cold, cough, allergy, bronchodilator and anti-asthmatic OTC drugs.
Mucinex Sinus saline nasal spray is designed to treat sinus-related symptoms as “first-ever drug-free saline product” with a dual nozzle offering two spray settings, marketer Reckitt-Benckiser says.
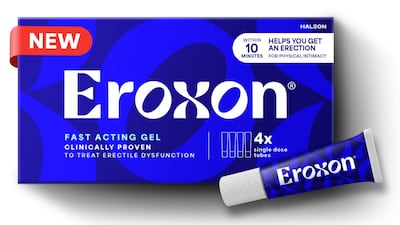
October will see the bricks-and-mortar launch of the first FDA-approved OTC treatment for erectile dysfunction in the US by Haleon. HBW Insight catches up with the company's North America president Lisa Paley to find out what Haleon has planned for Eroxon's arrival in stores.
FDA approves Flumist consumers can use at home for prevention of flu caused by virus subtypes A and B in individuals 2 through 49 years old in a proposal submitted by AstraZeneca’s MedImmune.
Bayer’s Midol and nonprofit Period. provide PeriodTalk campaign for awareness of the gaps in period education; “The M Factor” film coming day before World Menopause Day in October; Exeltis USA’s Blues Away provides postpartum mood support; and Doctor's Best Women's Collection includes menopause, sleep, digestive, heart and hair/skin/nails support products.