Rx-to-OTC Switch
Final rule’s requirement that ACNU formulations also remain available as Rx generics could limit revenues for marketers of the nonprescription drugs while approvals of ACNU applications from studies with participants having drugs delivered to their homes may require marketers to limit distribution to direct-to-consumer without clearance for sales in retail stores.
Study completed in 2024 showed consumers using an online application accurately self-selected and safely used an Rx statin drug. An “ACNU” switch NDA would have to first show conventional OTC labeling won’t support proper self-selection and use.
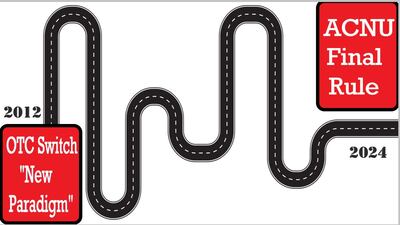
Agency’s proposal for targeting making more formulations available nonprescription through “novel switches” went through changes on its path from idea in 2012 to proposed rule in 2022 and final rule published in December.
FDA’s ‘ACNU’ rule went through numerous twists and turns from agency’s “new paradigm” idea of OTC switches in 2012 to publication.
Oral pseudoephedrine decongestants marketed by Kenuve, Haleon and Opella, amongst others, are now only available with a doctor's prescription in France.
OTC antifungal fluconazole will soon only be available with a doctor's prescription in Norway.
Consumers in Denmark no longer need a prescription to buy opioid antagonist naloxone as the country looks to tackle the growing issue of overdose.
FDA’s OTC office director details a 2023 guidance as opening doors for NRT innovation at recent public meeting, but researchers and an industry executive note the most recent approval in the US for an innovative NRT was more than 20 years ago and say FDA isn’t allowing sufficient flexibility for approvals of new products or indications other than cessation related to quitting smoking.
Even at conservative estimates, switching an additional 97 molecules could save about €200m, according to France's consumer health industry association.
Biden’s order for proposed rules could boost support for Democrat Kamla Harris from undecided voters concerned about reproductive rights or for Republican Donald Trump from those who agree with his conservative stance on access to birth control, which he argues should be up to states to regulate. Proposed rules also could stir consumers not planning to vote to go to the polls.
Qnovia notes NRT inhalation product recently received investigational new drug clearance from FDA as agency and NIH say innovation needed smoking cessation to help improve rate of success for quitting the habit that kills around 500,000 US consumers annually.
UK consumer healthcare industry association PAGB is calling on the UK government to explore the Rx-to-OTC switch of at least 25 OTC products over five years, and work with the country's medicines agency to re-evaluate the restrictive criteria for reclassification.
In addition to commentary and information about the OTC drug, dietary supplement and cosmetic industries, speakers offered candid remarks on subjects well known across the consumer health industry or well recognized by consumers.
“It's really the elected officials who are making the final determination on what happens. They're really the owners of the teams, you guys in the industry are playing the game we’re refereeing,” FDA Commissioner Robert Califf says at Consumer Healthcare Products Association conference.
An end to litigation filed by institutional investors, insurance companies and pension funds comes four CEOs after Perrigo's chief exec during Mylan's tender in 2015, Joseph Papa, resigned to lead another firm in 2016. Plaintiff attorneys are asking for up to 20% of settlement in fees.
Proposal to have panelists rate the strength of efficacy and safety evidence on a numeric scale also draws support in written comments on ways to optimize the advisory committee process.
David Ball moves from Bayer’s North America business to be Perrigo’s first chief brand and digital officer. He joins CEO who also moved to Perrigo from Bayer with decades of branded product experience.
A draft declaration to be presented to the UN General Assembly in September suggests that a “lack of regulation of over-the-counter use of antimicrobials” is one of the “drivers of antimicrobial resistance.” Industry, however, insists that misuse and over-prescription of antibiotics are the primary drivers of AMR, and is advocating for the text to be amended accordingly.
Germany's Expert Committee on Prescription recommends the Rx-to-OTC switch of an azelastine and fluticasone propionate combination nasal spray, the second time around. Low-cost smoking cessation drug cytisine, on the other hand, is denied - also the second time it has appeared before the committee.
Agency used a 10-year average with updated figures to calculate FY2025 PDUFA application fee and limit impact of submission volatility, but still allowed GDUFA and BsUFA fees to skyrocket.