Policy & Regulation
FDA Office of Nonprescription Drugs explains in its proposed commitment letter for fiscal years 2026-2030, based in part on suggestions by stakeholders, that it will heighten its attention to collecting those fees.
The Washington State Department of Ecology publishes ‘Interim Policy on Lead in Cosmetics’ which provides safe harbor options for cosmetic products struggling with the 1ppm limit under the state’s Toxic Free Cosmetics Act, while the department gathers information under a newly opened rulemaking to ‘identify a feasible approach to regulating lead in cosmetic products.’
Biovanta’s products relieved symptoms faster than OTC drugs available under FDA monograph, according to clinical trial by brand owner Applied Biological Laboratories. Chief scientific officer Nazlie Latefi said the results were “greatest decreases to date in overall illness severity using clinically validated measures and comparable study design.”
A recent Kenvue survey shows that an overwhelming majority of UK pharmacists believe many health issues could be prevented through better self-care.
Business
A round-up of the latest European consumer health appointments: Maxwellia adds CFO and board member; Reckitt restructures operations; Barentz names CEO.
Perrigo's retail customers are expecting real "net zero" progress from consumer health manufacturers, which is in turn driving the firm's sustainability ambitions and expectations from their own suppliers, Perrigo’s UK ethical compliance lead Isobel Gay tells HBW Insight in this exclusive interview.
Haleon now holds 88% of its China OTC joint venture after acquiring a larger stake in the business from its local partners.
National Advertising Division found multiple faults with Oral Essentials’ evidence for claim, including that an in vitro study the firm conducted on “mouse cells is not dispositive of whether the same product would have any adverse effects on humans under real world conditions.”
Innovation & IP
Bayer Consumer Health snaps up digital therapeutic Cara Care for its Precision Health business with an eye to developing its self-care capabilities.
Bayer Consumer Health shelves European launch of topical OTC onychomycosis treatment under its Canesten brand after a Phase III trial of the product - developed by Sweden's Moberg Pharma - failed to meet its primary endpoint.
Winners across 12 categories were announced at the OTC Marketing Awards 2024 on 21 November, with Opella taking the top prize as OTC Company of the Year.
Consumers in Denmark no longer need a prescription to buy opioid antagonist naloxone as the country looks to tackle the growing issue of overdose.
Rx-to-OTC Switch
Final rule’s requirement that ACNU formulations also remain available as Rx generics could limit revenues for marketers of the nonprescription drugs while approvals of ACNU applications from studies with participants having drugs delivered to their homes may require marketers to limit distribution to direct-to-consumer without clearance for sales in retail stores.
Study completed in 2024 showed consumers using an online application accurately self-selected and safely used an Rx statin drug. An “ACNU” switch NDA would have to first show conventional OTC labeling won’t support proper self-selection and use.
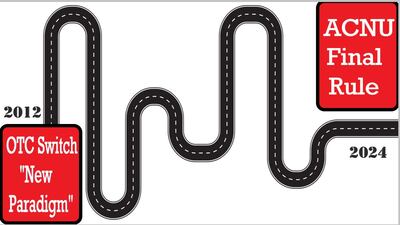
Agency’s proposal for targeting making more formulations available nonprescription through “novel switches” went through changes on its path from idea in 2012 to proposed rule in 2022 and final rule published in December.
FDA’s ‘ACNU’ rule went through numerous twists and turns from agency’s “new paradigm” idea of OTC switches in 2012 to publication.