Interviews
Perrigo's retail customers are expecting real "net zero" progress from consumer health manufacturers, which is in turn driving the firm's sustainability ambitions and expectations from their own suppliers, Perrigo’s UK ethical compliance lead Isobel Gay tells HBW Insight in this exclusive interview.
Kenvue's EMEA and LATAM head Carlton Lawson reflects on the firm's 18 months of independence, pointing to significant investments and operational improvements as key milestones on its spin out journey so far.
Xytogen Biotech’s Factorfive stem cell-based skin care delivers benefits that are greater than the company claims, says founder and CEO John Aylworth. Available mostly through clinicians’ offices, the formulas are based on a proprietary process that triggers stem cells to send out growth factor signals, which are captured in a ‘conditioned media’ and used as the basis for products.
Tariffs are expected again under a second Trump Administration and the future of the Americas Partnership for Economic Prosperity is uncertain, says the Personal Care Products Council, which will continue to push for regulatory alignment and lower trade barriers.
Bayer Consumer Health is replacing mixed material PVC blister packs with monomaterial PET blisters beginning with Aleve in the Netherlands. HBW Insight speaks to vice president and global head of design, packaging, product experience and sustainability, Chris Padain, about the "evolutionary" strategy behind the move, the complexities of choosing the right material for the right product, and the importance of industry cross-collaboration via associations like GSCF.
EFSA has published new guidance to assist companies navigating the EU’s novel food authorisation process. HBW Insight speaks to experts Dr Mari Eskola of Medfiles and Dr Jérôme Le Bloch of FoodChain ID who offer differing views on the benefits and drawbacks of the guidance for industry.
Cosmetics Europe’s John Chave highlights the important role that industry must have in educating the public about chemicals used in cosmetic products. The association’s COSMILE Europe database launched last year is one such measure to combat misinformation while industry braces for potential impacts resulting from new hazard categories under the Classification, Labeling and Packaging (CLP) regulation.
In addition to its recently established Precision Health division, Bayer Consumer Health is using artificial intelligence to save time on routine tasks and to manage supply and demand, the firm’s head of digital transformation and IT, Cristina Nitulescu, tells HBW Insight.
Cosmetics Europe hopes a more “competitive” spirit will define the 2024-2029 regime. Director-general John Chave discusses the association’s outlook on planned revisions to REACH and the Cosmetic Products Regulation in the new EU institutional mandate.
Genetic Analysis CEO Ronny Hermansen and Christina Casén, senior VP of clinical and medical affairs, discuss the company’s polymerase chain reaction (PCR)-based approach to gut microbiota profiling versus DNA sequencing, competitive landscape including DTC, and opportunities for supporting pharma R&D and assessing drug treatment success.
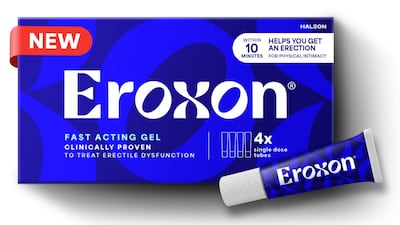
October will see the bricks-and-mortar launch of the first FDA-approved OTC treatment for erectile dysfunction in the US by Haleon. HBW Insight catches up with the company's North America president Lisa Paley to find out what Haleon has planned for Eroxon's arrival in stores.
This Thursday (26 October) the United Nations will discuss whether OTC antimicrobial products should be restricted to prescription-only status by UN member states. In advance of this, we catch up with UK association CEO Michelle Riddalls, to learn about the PAGB’s role in crafting the global consumer healthcare industry’s response.
Escaping A Regulatory Labyrinth: France Adopts Streamlined Approach To Food Supplement Notifications
Companies launching food supplements in France must now navigate a new product notification platform. EcoMundo’s Corinne Rayapin discusses why the change is happening and the expected benefits for both industry and consumers.
In this episode of HBW Insight's Over The Counter podcast, we connect with Neil D’Souza, CEO of Makersite GmbH, a data software company based in Stuttgart, Germany that offers “next generation” product data management tools that help personal care and other industries manage product sustainability, cost and compliance.
In this instalment of HBW Insight’s “Inside Regulatory Affairs” series, AdverCheck managing directors Lucy Rochford and James Walmsley describe how the consumer health industry is increasingly outsourcing its advertising copy review as it moves closer to the fast-moving consumer goods sector. They also point to a worrying trend in the UK of advertising prescription-only medicines online.
Taking over from the son of the company’s founder at the beginning of 2023, Martin Arès, the CEO of Canadian generics manufacturer Pharmascience, talks to Generics Bulletin about investment, international expansion and biosimilars.