‘Lack Of Understanding’ Here, Spiked Product There Add Up To US Supplement Industry Image Problem
Executive Summary
Research suggesting FDA more stringently regulate claims for fish oil supplements impresses industry as another sign the agency’s regulation and the requirements fulfilled by compliant businesses aren’t appreciated or understood. FDA announcement of ED drugs in products labeled as supplements shows agency must be aware students are starting or returning to classes.
Recent unrelated events provide the latest evidence that the US Food and Drug Administration and responsible firms face long odds against making the dietary supplement market trusted by consumers and scientists alike.
What’s more, finding undeclared erectile dysfunction drugs in products labeled as dietary supplements shows the FDA must be aware that high school and college classes are starting around the country.
On the other hand, the Journal of the American Medical Association’s publication of research suggesting the FDA more stringently regulate label claims for fish oil supplements impresses industry stakeholders as merely another among the routine signs that the agency’s regulation of the industry and the requirements fulfilled by compliant businesses aren’t appreciated or understood by some medical experts as well as other learned persons across fields of research.
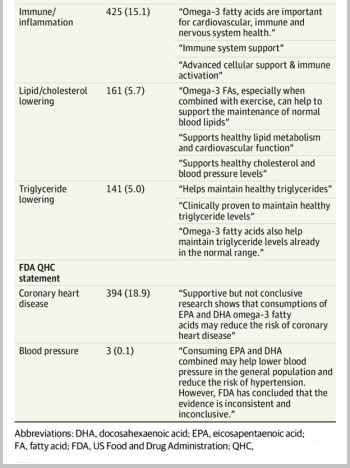
The JAMA Cardiology cross-sectional study of 2.819 fish oil supplements found 73.9% made at least one health claim, usually related to heart health, followed by brain and joint health, and FDA-approved qualified health claim language was infrequently used (see table ).
The total daily dose of eicosapentaenoic acid plus docosahexaenoic acid “was highly variable between supplements,” according to the researchers led by Ann Marie Navar, University of Texas Southwestern Medical Center, Dallas.
Navar and her colleagues said the results show “a lack of trial data showing efficacy” supporting health claims on the majority of fish oil supplement labels, usually structure/function claims, “that imply a health benefit across a variety of organ systems.”
Omega-3 Supplement Label Claims
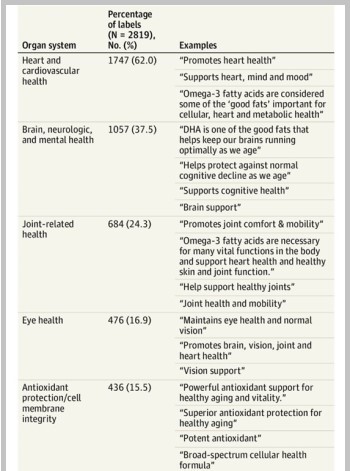 Heart/cVD claims, including QHCs, were most common on omega-3 supplement labels reviewed in study PUBLISHED IN JAMA CARDIOLOGY, followed by brain and joint health claims; No labels noted potential risk of atrial fibrillation.
Heart/cVD claims, including QHCs, were most common on omega-3 supplement labels reviewed in study PUBLISHED IN JAMA CARDIOLOGY, followed by brain and joint health claims; No labels noted potential risk of atrial fibrillation.
“Significant heterogeneity exists in the daily dose of EPA+DHA in available supplements, leading to potential variability in safety and efficacy between supplements. Increasing regulation of dietary supplement labeling may be needed to prevent consumer misinformation,” wrote Navar, who along with one of her colleagues reported receiving grants or personal fees, unrelated to this study, from multiple pharmaceutical and medical technology firms.
Researchers’ Conclusion ‘Predictable And Unsupported’
The Council of Responsible Nutrition’s response to the JAMA Cardiology study wasn’t its first rodeo with published research critical of the supplement industry and the FDA.
“The call for ‘additional regulation of dietary supplement labeling’ is both predictable and unsupported by the research,” said president and CEO Steve Mister in the trade group’s announcement.
Mister added that the “study demonstrates an amazing lack of understanding of the many different reasons why consumers choose to use supplements for better health” and “there is nothing in this study that should persuade consumers to change their omega-3 regimens for better health.”
The CRN also stated structure/function claims are a type of claim permitted for dietary supplements with support from scientific evidence and are regulated by the FDA under a different standard than qualified health claims. But the study “appears to ignore” that the different types of claims “serve different purposes—one to provide general non-disease specific health information to consumers” and he other “permitted to discuss the relationship between a nutrient and disease risk.”
The CRN and other trade groups and other industry stakeholders previously have been prompted to make similar responses to numerous published studies questioning the efficacy both of supplements and of the FDA’s regulation of the industry.
For instance, supplements containing melatonin – formulated as synthetic copies of a hormone occurring naturally in the human body and long a lawful sleep-support dietary ingredient – were scrutinized by medical researchers in a JAMA report published in March because labeled amounts of the ingredient don’t match levels found in the products. (Also see "Melatonin Dose Study, Steroid Warning Show Both Sides Of Coin For US Supplement Industry Image" - HBW Insight, 26 Apr, 2023.)
However, while almost all the 25 sampled products contained adult servings and were labeled for use by adults, the authors used pediatric data in their findings. Additionally, the study applied the FDA’s drug standard for overage/underage of ingredient levels, which is +/- 10 percent, but for supplements the agency allows a different measure.
Moreover, the mere marketing of omega-3 supplements specifically was challenged in an International Trade Commission complaint by Amarin Inc., which also has filed civil complaints and used an industry self-regulation forum attempting to stop supplement firms from comparing their fish oil products to its fish oil-derived drug Vascepa indicated for cholesterol reduction. (Also see "US Omega-3 Supplement Firm Changes Claims On Amarin Challenge, But Not Label Or Company Name" - HBW Insight, 26 Apr, 2022.)
‘Big Guys,’ ‘WeFun’ Spell Beware
The FDA’s latest alert to consumers about products labeled as supplements but found to contain erectile dysfunction drugs is a six-item list noting products, some identified as energy drinks, with brands including “Big Guys” and “WeFun.” Like nearly all drug-spiked supplements, the latest products the FDA warns about were sold online, including through Amazon Inc.’s e-commerce platform.
The product names change but the problems don’t when it comes to the FDA’s monitoring for undisclosed drug ingredients in products labeled as supplements available to US consumers. If it’s not ingredients which are copies of approved ED drugs, the agency’s finding drugs for weight loss and strength training which aren’t approved for use in any product available in the US and which, like any drug, are unlawful for use in supplements.
 products labeled as supplements but containing ed drugs recently found online by FDA include big guys extreme power, above, and wefun, below.
Source: FDA
products labeled as supplements but containing ed drugs recently found online by FDA include big guys extreme power, above, and wefun, below.
Source: FDA
DSHEA, again, is the source for the prohibition on using drug ingredients in supplement. But it’s also the reason businesses intentionally or unintentionally violating that ban are making available to US consumers products labeled as supplements but containing ED and other drugs.
With no regulatory barrier to market entry other than what has been proven to be the largely unenforceable new dietary ingredient notification requirement, businesses large and small, through intentional or unintentional violations, can offer for sale products labeled as supplements but containing undeclared drugs or other substances FDA regulations prohibit.
Businesses also can make obviously violative health claims for supplements, including for products which might otherwise be compliant with FDA regulations.
Detecting noncompliant label and advertising claims is less complicated and resource-intensive for the FDA than determining whether so-called supplement products contain drugs. And that’s fortunate because violative claims likely are far larger in number than supplements containing drugs.
No Relief From Drug-Spiked Supplements
However, finding supplements containing drugs is a constant burden for the FDA. Supplement manufacturers and marketers compliant with FDA regulations could argue that those products are an even larger headache for them.
Every time the FDA makes an announcement like its latest alert about the six products found to contain ED drugs, compliant industry would point out those products are labeled as but are not dietary supplements and that the large majority of supplements available to US consumers are from responsible firms and are safe.
On the other hand, every time the FDA makes this type of announcement, critics of the industry and of the agency’s oversight will point to still more evidence that tighter regulation is needed.
Supplement industry stakeholders support tighter regulation by way of the FDA more consistently exercising its authorities established by DSHEA, which they say are sufficient for effective oversight. Critics of the industry and the FDA, without acknowledging whether their proposals would require amending or even overhauling DSHEA, contend that additional rules are needed because the current framework isn’t effective.
The FDA, for its part, hasn’t championed amending DSHEA. Instead, in addition to violative claims and undeclared ingredients, it sees manufacturers and marketers as commonly falling short of compliance with the agency’s regulations, particularly for NDI notifications.
The agency has limited agreement from trade groups and other industry stakeholders about adding a mandatory product list rule, a requirement the FDA hopes would help it identify every supplement product available to US consumers. (Also see "US Consumer Health Industry In 2023: Mandatory Listing Has Supplement Sector’s Attention" - HBW Insight, 29 Jan, 2023.)
Stakeholders generally support the FDA producing a public-facing database of supplement products but oppose allowing the agency to use information firms submit for the list, or a firm’s failure to provide information, as evidence to launch regulatory actions.
The FDA also will point out that while the US supplement industry has grown from roughly $4bn annual sales in 1994 to estimates exceeding $80bn currently, the agency’s funding and other resources for regulating the industry haven’t grown enough. (Also see "The Dietary Supplement Market US FDA Is Regulating Isn’t Your 1994 DSHEA Version" - HBW Insight, 18 May, 2023.)
With additional funding, perhaps the FDA would more frequently alert consumers to products labeled as supplements but containing undeclared drugs.
European Union regulatory authorities also are finding supplements marketed to enhance sexual performance containing undeclared drug ingredients. (Also see "EU Authorities Clamp Down On Unsafe Erectile Dysfunction Supplements In Q2" - HBW Insight, 23 Aug, 2023.)
According to the FDA's “Health Fraud Product Database,” which it says “includes only a small fraction of the potentially dangerous products marketed to consumers online and in stores,” a 9 August entry about ED drug sildenafil found in supplement was the most recent alert after April and May alerts concerning additional supplements containing ED drugs as well as products with ingredients indicated for treating arthritis.
The database likely will grow late in the year, when the FDA typically reminds the supplement industry in an announcement that drug-spiked products labeled as supplements still are being found for sale online. (Also see "From: US FDA; To: Supplement Industry – Tis The Season To Note Disease Claims, Undeclared Drugs" - HBW Insight, 9 Dec, 2021.)
The latest alerts, rather than bringing yuletide greetings, arrive during the back-to-school season as young people return to or start classes.